Research
Intracellular Molecular Delivery
Delivering molecules into cells can be made more precise with vectors that direct intracellular cargo to particular compartments. Our work has focused on mitochondrial targeting with a versatile peptide based vector that promotes efficient cellular uptake and strong mitochondrial localization.
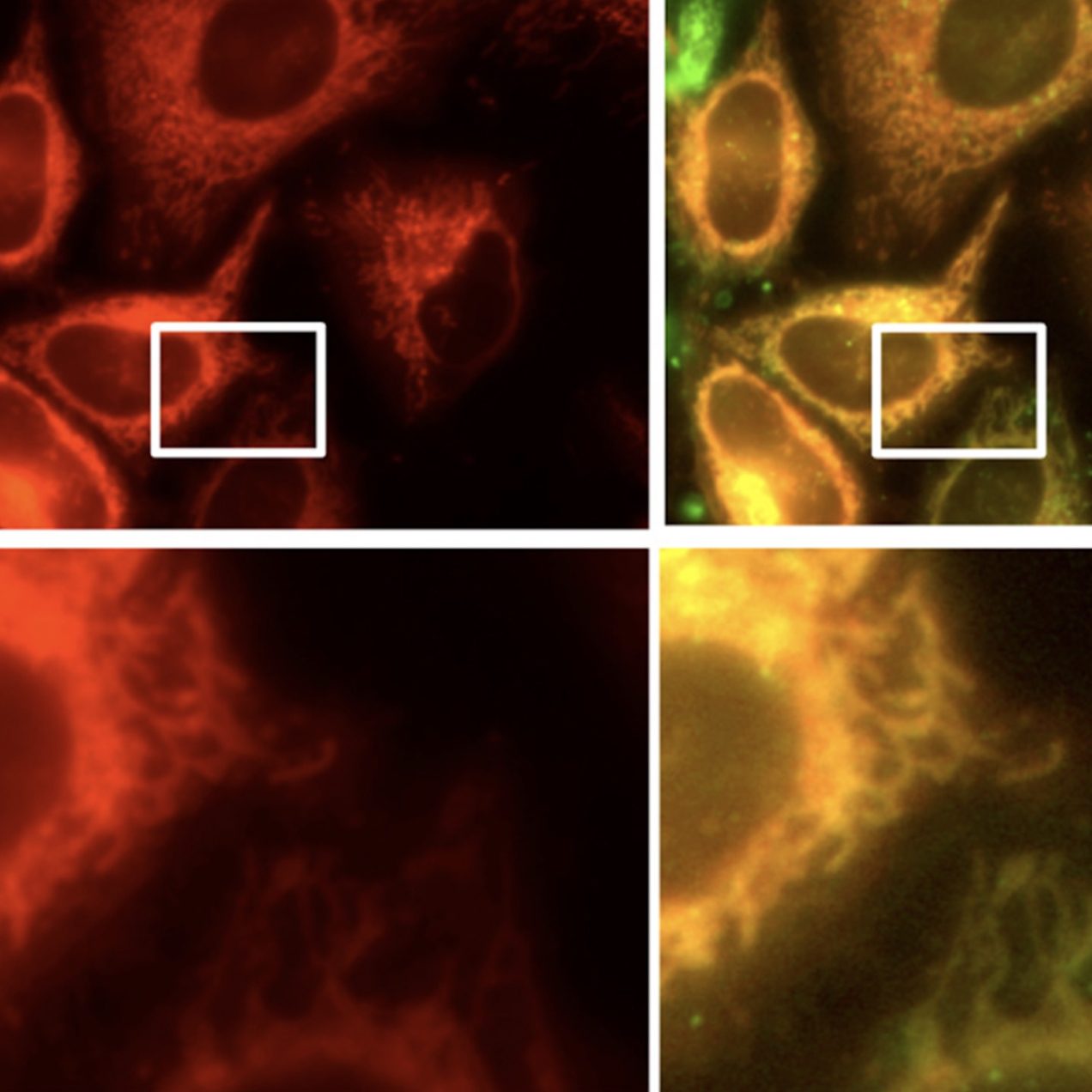
Mitochondrial Drug Delivery
Using a peptide-based delivery vector that can carry reactive cargo into the mitochondria of live mammalian cells, we have delivered clinically-utilized anticancer drugs that typically act within the nucleus. This approach has elucidated a variety of interesting activities for mitochondrially-localized probes. We’ve also shown that mitochondrial delivery can detoxify antimicrobials within human cells by sequestering the drugs within this organelle.
Featured Publications
“Delivery and Release of Small-Molecule Probes in Mitochondria Using Traceless Linkers.”,
Journal of the American Chemical Society, 2017, 139, 9455-9458.
“Targeting Mitochondrial DNA with a Platinum-Based Anticancer Agent."
Cell Chemical Biology, 2013, 20, 1323-1328.
"Targeted Delivery of Doxorubicin to Mitochondria." G. Chamberlain, D.V Tulumello, S.O. Kelley
ACS Chem. Biol., 2013, 8, 1389-1395.
"Maximizing the Therapeutic Window of an Antimicrobial Drug by Imparting Mitochondrial Sequestration in Human Cells "
J. Am. Chem. Soc., 2011, 133, 3260.
"Re-routing Chlorambucil to Mitochondria Enhances Potency and Combats Drug Resistance in Cancer Cells"
Cell Chemical Biology, 2011, 18, 445.
"Mitochondria-Penetrating Peptides."
Cell Chemical Biology,, 2008, 15, 375.
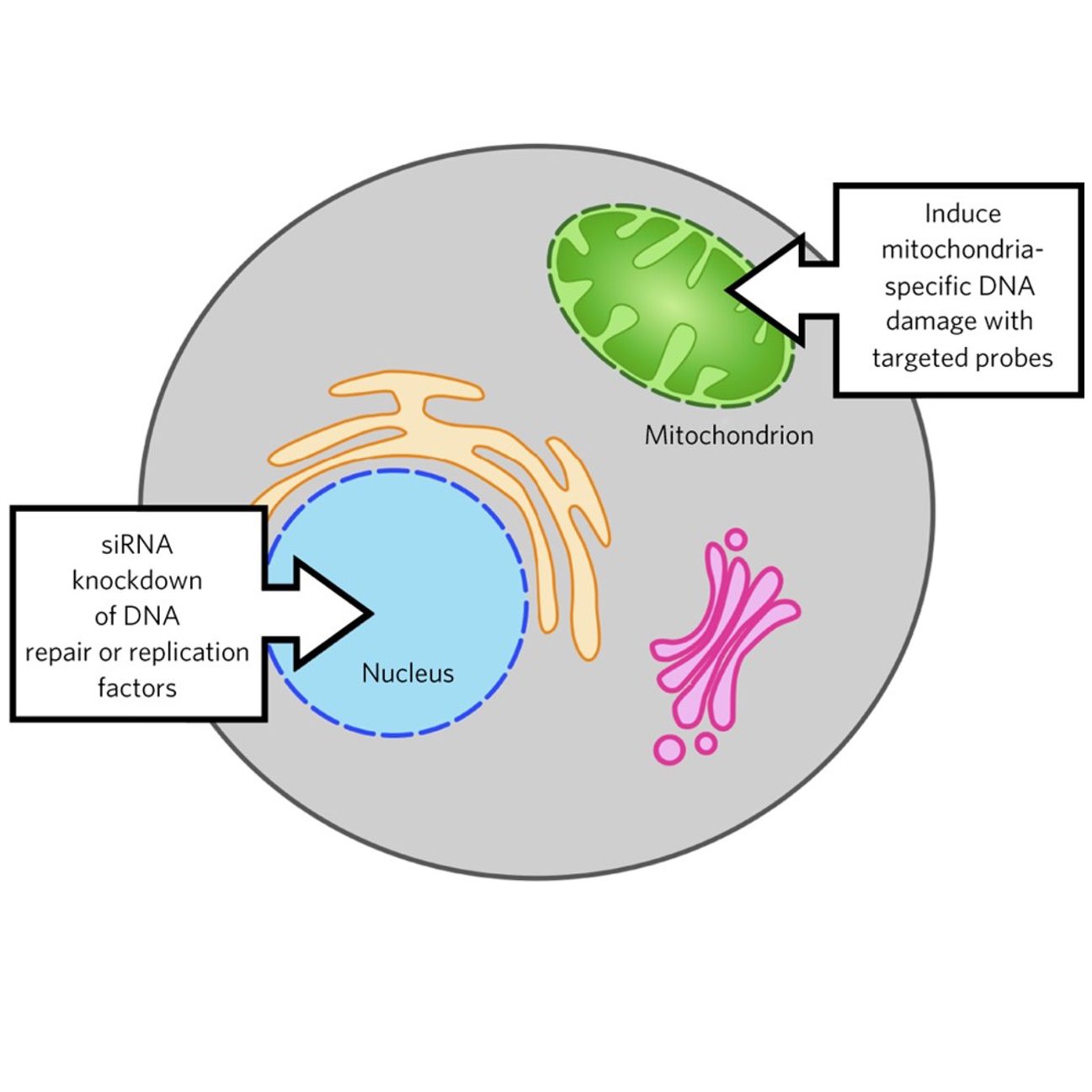
Mitochondrial Probes for the Discovery of New Organelle-Specific Activities
Mitochondrial probes also enable high-throughput screening for new organelle-specific factors. Using siRNA or CRISPR/Cas-based screening to knockdown factors in the nucleus that may function in mitochondria, synergistic effects can be detected in the presence of a mt-targeted probe. This type of analysis has allowed us to identify new factors that are key players in mitochondrial function.
Featured Publications
"Mitochondrial DNA Repair and Replication Proteins Revealed by Targeted Chemical Probes."
Nature Chemical Biology, 2016, 12, 567-573.
"Mitochondrial Chemical Biology: New Probes Elucidate the Secrets of the Powerhouse of the Cell."
Cell Chemical Biology, 2016, 23, 917-927.